Xinxing Zhang, of Johns Hopkins University in Baltimore, was investigating the synthesis of novel mixed metal hydrides using a pulsed arc cluster ionisation source. Here electrodes of platinum and zinc were subjected to repetitive electrical discharge in a vacuum, with hydrogen allowed to bleed in. As the metal vaporises and the hydrogen molecules dissociate, a high energy soup of reactive species is created, which is then rapidly cooled by helium causing the various species to form chemical bonds in the most energetically favourable configuration. ‘We are not directing the reaction – nature decides what is the most stable outcome,’ says Kit Bowen, the team leader.
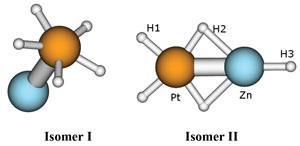
The products of the process were separated by time-of-flight mass spectrometry, and one particularly stable cluster anion was observed, containing one platinum, one zinc and five hydrogens. This anion was interrogated by photoelectron spectroscopy, which yields information about the binding energy of the electrons in the anion.
This information was passed to theoretical chemist Anastassia Alexandrova at the University of California, Los Angeles. Alexandrova was able to work out how the structure is configured: the zinc is attached to the platinum, which in turn has five hydrogens arranged in a cycle around it. This H5 cycle is stabilised by s-aromaticity, mediated by the platinum. The structure resembles a mushroom with a flattened head consisting of the hydrogen ring.
Experimental confirmation
The idea of aromaticity arising from s bonds has been given serious consideration only in the last 15 years or so. It is known to exist in the H3+ ion, which is abundant in interstellar space, and has been theorised to exist in an H5- system. However, no-one has been able to demonstrate this experimentally until now.
‘This is the first observation of aromaticity in a metal hydride, and the first time as far as I know that platinum has exhibited this fivefold planar co-ordination,’ says Alexandrova. ‘I think it is likely that this type of aromaticity will be occurring much more widely than previously thought. It is important because when we think about the design of, say, transition metal catalysts that form hydrides along the catalytic pathway, this type of electronic interaction can play an important role in the stability and reactivity of these species. The stability conferred by aromaticity can count towards or against the kinetics of the process.’
Experts on chemical bonding are impressed by the study. ‘I found it to be a very interesting result, and actually a very surprising one,’ says Lai-Sheng Wang of Brown University, US. ‘Usually in organic aromatic compounds, hydrogen atoms are just spectators, such as in benzene.’ Wang believes that the finding is important because of the role of transition metals in catalysis and hydrogen storage. ‘More stable hydrides may be discovered based on this new understanding of hydrogen–metal chemical bonding,’ he says.
Alexander Boldyrev of Utah State University in the US, say that he is ‘not aware of any other example of such unusual coordination’. The mixed-metal hydride cluster is, he says, ‘An interesting example of an aromatic species and further extends aromaticity beyond organic chemistry.’






No comments yet