Enzyme-inspired ligand distinguishes between simple alkyl groups
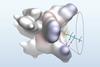
Designer ligand creates active site-like pocket for asymmetric radical reaction
A new catalyst promotes an asymmetric radical reaction using an engineered ligand that can differentiate between very similar alkyl groups. The stereoselective amination process forms highly enantioenriched α-chiral alkyl amines, a common motif in drug molecules.
Radicals are a common intermediate in many synthetic transformations. Their single electron makes these species extremely reactive, enabling rapid reactions with minimal energy input. However, for prochiral substrates – those with three different groups attached to the radical centre – controlling the enantioselectivity of the subsequent mechanistic steps is an almost impossible challenge.
