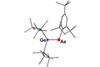
First arsenic–germanium double bond made
Scientists use push–pull substitution to synthesise heavy imine
Arsagermene, the first isolable compound containing an As=Ge double bond has been reported by international scientists.

Scientists use push–pull substitution to synthesise heavy imine
Arsagermene, the first isolable compound containing an As=Ge double bond has been reported by international scientists.