First ever triply bridging phosphine glows in two colours when crushed
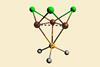
Copper triangle topped with μ3-bridging phosphorus ligand shows unusual colour-changing mechanochromic luminescence
The first triply bridging phosphine ligand that tops a crown of three copper atoms has been made by chemists in Russia. They also discovered that the compound has unusual luminescent properties, changing from glowing white–blue to green when ground up in a mortar and to orange when cooled to –118°C.
