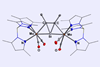
First flat tetravalent silicon stabilised by metals and aromaticity
Chemists make stable compound featuring a silicon atom that forgoes tetrahedral geometry
Silicon has entered flatland with the first stable compound featuring a planar – rather than tetrahedral – tetracoordinated silicon atom. The unusual molecule was made possible by stabilisation coming from two metal centres as well as an aromatic core.