First uranium–rhodium bond shows that shorter is not stronger
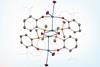
Complex with one of the shortest uranium–transition metal bonds ever reported is unstable in solution

Complex with one of the shortest uranium–transition metal bonds ever reported is unstable in solution