UK chemists have used a combination of flow chemistry methods with solid-supported scavengers and reagents to synthesise the active pharmaceutical ingredient, imatinib, of the anticancer drug Gleevec. The method avoids the need for any manual handling of intermediates and allows the drug to be synthesised in high purity in less than a day.
Gleevec, developed by Novartis, is a tyrosine kinase inhibitor used for the treatment of chronic myeloid leukaemia and gastrointestinal stromal tumours. The drug molecule represents a particularly challenging target for flow chemistry because of the low solubility of many of the reaction components required for its synthesis. The team devised a new synthesis route that prevents the equipment blockages from product precipitation and avoids many of the labour and time intensive practices of traditional batch-based preparation.
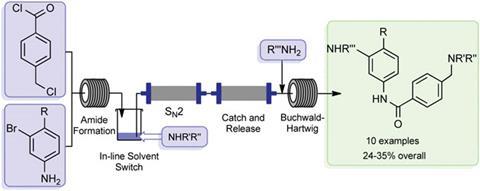
Unlike the conventional industrial synthesis of Gleevec, this newly developed route couples molecular fragments in a modular approach. Thomas Wirth, who works on microreactor technology at Cardiff University, UK, remarks that ‘although not designed to compete with the industrial synthesis, the modular approach allows an easy variation of building blocks for the efficient and rapid generation of Gleevec analogues for screening purposes’.
A total of nine analogues were synthesised using the final equipment set-up, which were then screened for anticancer activity. The findings revealed that the piperazine group in the drug molecule plays a role in receptor binding, rather than simply acting as a solubilising group as previously thought.
Ley's team is now working to combine the synthesis and screening to provide information on products rapidly, as well as extending their approach to new functional materials.






No comments yet