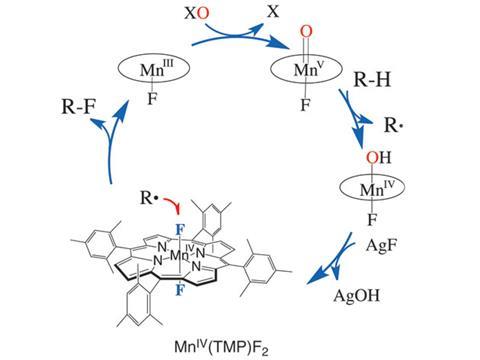
Despite fluorine’s popularity with medicinal chemists, reliable activation of aliphatic C–H bonds for fluorination has remained tantalisingly out of reach. Now, a manganese porphyrin has been used to selectively fluorinate sp3 carbon–hydrogen bonds using silver fluoride as a source of fluorine.
The manganese (III) porphyrin complex is oxidised by iodosylbenzene to form an oxomanganese (V) intermediate that selectively cleaves the C–H bond. The resulting carbon-centred radical reacts with a trans-difluoro manganese (IV) porphyrin, forming the C–F bond and regenerating the manganese (III) catalyst. The team of chemists, led by John Groves at Princeton University, US, used this method to selectively fluorinate alkanes, terpenoids and simple steroids.
Fluorine is particularly useful in drug design as it can prevent metabolism by cytochrome P450 enzymes and improves binding affinities. Isotopically labelled fluorine-18 can also be used in compounds for Positron Emission Tomography (PET) scans.






No comments yet