Fungi eat up old batteries and spit out metals
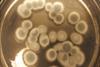
Lithium and cobalt can be recycled from worn out lithium-ion batteries in low energy process thanks to fungal acids

Lithium and cobalt can be recycled from worn out lithium-ion batteries in low energy process thanks to fungal acids