Boron's rich polyhedral structural chemistry continues to excite chemists, as German researchers find new ways to predict the relative stabilities of heteroboranes.
Boron?s rich polyhedral structural chemistry continues to excite chemists, as German researchers find new ways to predict the relative stabilities of heteroboranes.
The overall shapes of boron hydrides and carboranes (mixed hydrides of boron and carbon), indeed clusters in general, have been predictable for decades from rules devised by Bob Williams, Mike Mingos, Ken Wade and others, based on formula type and the number of electrons in the skeletal framework. However, working out which skeletal atoms go where, requiring coordination numbers and identities of neighbouring atoms to be considered, is harder than shape prediction.
What Farooq Kiani and Matthias Hofmann have done, by calculations on a range of heteroboranes, is to extend the use of ?energy penalties? for undesirable coordination numbers and neighbours to predict which isomers are the most stable, and the stability order of others. So simple calculations can give the kind of information only previously attainable from many experiments or long computations.
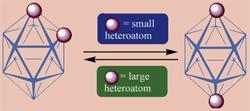
They found that smaller atoms tend to occupy positions away from each other in icosahedral heteroboranes, whereas larger atoms end up next to each other (see graphic). Ken Wade, of the University of Durham, said the work ?confirms and extends Williams?s predictions about the relative stabilities of isomers, and sheds new light on where and why the old generalisation that heteronuclear bonds are likely to be preferred to homonuclear bonds has its limitations?.
Hofmann, of the University of Heidelberg, commented ?we wanted to expand the earlier qualitative rules underlying the cluster buildup by quantitative rules ? and succeeded?. However, he went on, ?so-called macropolyhedra composed of fused smaller cluster fragments are still a big challenge?.
Neil D Withers
References
F A Kiani and M Hofmann, Dalton Trans., 2006 (DOI: 10.1039/<MAN>b512700a</MAN>)






No comments yet