A strain of engineered Escherichia coli blends two forms of metabolism, enabling the bacterium to work with previously unusable carbon sources to help displace fossil fuel-based production of chemicals.
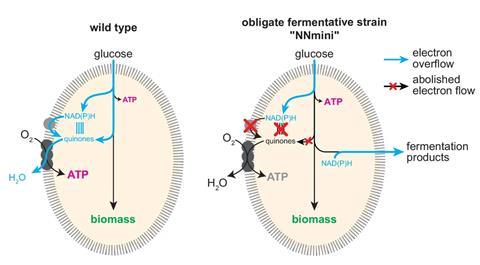
In nature, E. coli builds molecules from sugars using fermentation or respiration. In respiration, electrons freed by the breakdown of sugars are released and accepted by oxygen. As fermentation is anaerobic, freed electrons are instead packaged into metabolites, which are then excreted. However, this requires a balance between the number of electrons in the substrate and product, limiting the types of molecules available for fermentation.
‘Fermentation processes are all based on sugars and that competes with food production,’ explains Steffen Lindner-Mehlich, a biochemist at the Charité University Hospital in Berlin and lead author of the new research. To unlock the use of sustainable feedstocks such as agriculture waste Lindner-Mehlich and his group engineered the first E. coli strain capable of what they call respiro–fermentation. This new metabolism allows bacteria to use oxygen as an electron acceptor during fermentation.
Metabolic rewiring
The first step was removing all the genes required for respiration. This forces the cells to use fermentation. Then they selectively reinserted genes coding for enzymes that direct electrons to specific parts of the respiration pathway. The result is cells that ferment but are not constrained by balancing electrons.
In tests the team fed the bacteria glycerol, a previously unfermentable carbohydrate, and produced lactate, a natural fermentation product. Confirming that unbalanced fermentation was possible they next swapped the genes responsible to produce lactate with genes to produce isobutanol, an alcohol used in the production of several commodity chemicals. The cells again performed unbalanced and aerobic fermentation into the desired product.
‘This type of radical metabolic rewiring is an elegant example of how central metabolism could be engineered as opposed to the most classical approach of a few genes being deleted or overexpressed,’ says Pablo Iván Nikel, a biotechnologist at the Technical University of Denmark.
Jens Nielsen, a systems biologist at Chalmers University of Technology in Sweden, agrees this is a positive demonstration of synthetic biology. However, he questioned the use of glycerol as a feedstock. ‘[Glycerol] is not abundant and only available due to a heavily subsidised biodiesel industry that will probably not survive long term,’ he says.
According to Jan Krüsemann, co-author and PhD student in the Charité group, their method is highly customisable. ‘Glycerol is cheap right now,’ he points out. But as market forces and substrate availability change ‘then we can use our variation of the strain that uses acetate or we use the one that uses methanol’, Krüsemann explains.
Further optimisation and scaling are required. ‘Titres can be improved,’ says Nikel. ‘It would be interesting to see what happens in a bioreactor, where oxygen transfer and mixing start to play a major role.’ According to Nikel, the study is ‘a solid proof of concept’. ‘Understanding the behaviour of the cells in a big tank, where multiple factors define metabolic lifestyles, will be the next obvious step towards optimisation,’ he adds.
Correction: This story was updated on 13 August 2024 to correct the spelling of the respiro–fermentation process.
References
H Schulz-Mirbach et al, Nat. Commun., 2024, DOI: 10.1038/s41467-024-51029-x










No comments yet