Hydrogen bonding increases the stability of tear film, new research shows.1 The findings could help illuminate the origins of dry eye syndrome and lead to new strategies for treating the condition.
Eyes are important organs that require protection from debris and bacteria. Located between the mucosal and meibomian layers, tear film is an aqueous layer that provides protection by covering the cornea’s surface. But when the tear film is discontinuous and unstable, dry spots can form leading to dry eye syndrome – a problem that affects millions worldwide.2
While ocular mucins are understood to stabilise and lubricate tear film, and an oily substance called meibum prevents evaporation, the role of electrolytes within tear film is unclear. Now, a research team surrounding Suraj Borkar of Stanford University in the US has investigated how these solutes effect film stability.
The study used white light interferometry to compare how solutions of sodium chloride and Hank’s buffer – which contains sodium, chloride, potassium and phosphate ions and glucose – responded when applied to a silica glass dome that mimicked the curvature of the cornea.
With the sodium chloride solution, the team saw salt crystals and dry spots form on the dome. But with Hank’s buffer solution – which the team used to represent tear film – these crystals were absent. Instead, there was a delay in evaporation and a local thickening of the film.
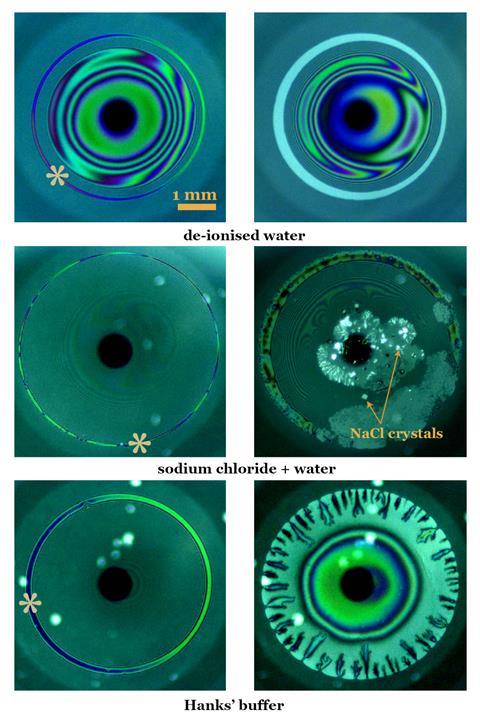
It is ‘surprising that it doesn’t take much buffer salt to really prevent’ the formation of salt crystals, comments Richard Braun, a mathematician who develops models to study tear film stability at the University of Delaware in the US.
Borkar and colleagues ascertained that the sodium chloride in Hank’s buffer contributes to the local thickening. It increases the surface tension of the film and subsequently creates a surface tension differential between the edges of the dome and the centre, leading to an influx of fluid towards the centre.
‘We saw something very fascinating when we had a buffered solution that is very close in its composition [to the tear film],’ notes Borkar – patterns known as viscous fingers, which were absent in the sodium chloride experiments. They deduced that hydrogen bonding from the hydroxyl groups in Hank’s buffer solution increases the viscosity of the film. The team linked this to Saffman–Taylor instability, when low viscosity fluid pushs against a high viscosity film results in viscous fingers. This observation paired with the local thickening resulted in increased film stability in the buffer solution compared with the sodium chloride solution.
The team hopes that better understanding the fundamentals behind tear film stability will advance treatments for dry eye syndrome.
References
1 S Borkar et al, Proc. National Acad. Sci., 2024, 121, e2407501121 (DOI: 10.1073/pnas.2407501121)
2 D Wróbel-Dudzińska et al, Int. J. Environ. Res. Public Health, 2023, 20, 1313 (DOI: 10.3390/ijerph20021313)














No comments yet