Hydrogens squeezed into record-breaking proximity
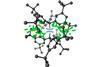
Shortest van der Waals interaction beats previous record holder by 0.051Å
The shortest interaction ever seen between hydrogen atoms bound to neighbouring molecules has been discovered by researchers in Germany.Chemists routinely draw lines between individual atoms to represent chemical bonds. However, chemical interactions are less static than these solid, dashed or dotted lines might imply – bonds can be twisted, stretched or compressed, depending on the molecules’ geometry.
