Intermediate considered ‘too reactive to isolate’ finally tamed
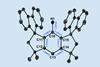
Nitrene species stabilised for three days with careful choice of substituent
A chemical intermediate once considered too reactive to isolate has been stabilised in the laboratory for three days by researchers in Germany. The team’s techniques enabled studies that were previously impossible due to the nitrene species’ fleeting nature and could allow researchers to further harness the molecules’ potential in synthetic chemistry.
