Iridium pincer complex promotes unprecedented ether decarbonylation
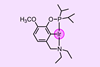
Scientists stumble upon unique transformation that involves breaking an extraordinary number of bonds
US researchers have demonstrated that an iridium pincer complex can stoichiometrically strip carbon monoxide from a wide range of ethers to give two hydrocarbyl products. The unique chain-rupturing transformation involves breaking an extraordinary number of bonds.