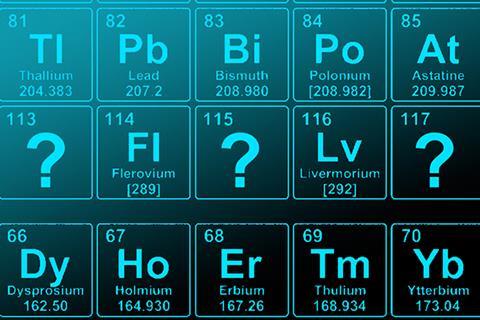
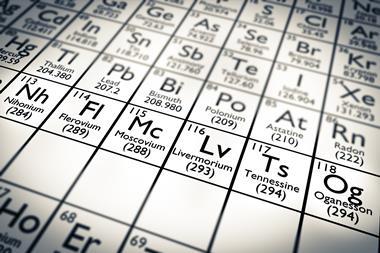
The proposed names for elements 113, 115, 117 and 118 are nihonium, moscovium, tennessine and oganesson respectively, the International Union of Pure and Applied Chemistry (Iupac) has announced.
‘It’s an exciting day for the world,’ says Lynn Soby, Iupac’s executive director.
The groups responsible for the discovery of these new elements each put forward their proposed name and symbol after Iupac confirmed their existence in January 2016. The criteria states an element may be named after a mythological figure or concept, geological place, scientist, elemental property, or mineral.
Nihonium (elemental symbol Nh) is the proposed name for element-113. The element was synthesised by Kosuke Morita’s group at RIKEN in Japan after they bombarded a bismuth target with zinc-70 nuclei in 2004 and 2012. Named after Japan, the element will be the first East Asian name to appear on the periodic table if ratified.
Scientists based in Russia and the US who discovered elements 115 and 117 have put forward the names moscovium (Mc) and tennessine (Ts), respectively. A collaboration between the Joint Institute of Nuclear Research in Russia and the Oak Ridge and Lawrence Livermore National Laboratories, US, elements 115 and 117 were both created in 2010. Both element names take their cues from geographical regions. Moscovium is named after Moscow, where the Joint Institute of Nuclear Research is based. Named after Tennessee, tennessine is a tribute to the region where a large amount of superheavy element research is conducted in the US.
The same group has also named element-118 oganesson (Og), in honour of the Russian nuclear physicist Yuri Oganessian who led the team that synthesised element-117.
The names will now be put up for public scrutiny in a five month consultation process before Iupac ratify the final names. ‘It’s important for people around the world to review the names to make sure that they fit with all the different languages,’ Soby tells Chemistry World. ‘Now the public and the scientific community can weigh in on things.’
Want to know more about how to make superheavy elements? Read our explainer here











No comments yet