Light-driven enzyme engineered and repurposed to catalyse unnatural reaction
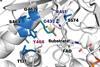
Directed evolution used to create photoenzyme that can perform new-to-nature radical cyclisation
A natural photoenzyme found in algae has been artificially evolved to perform an unnatural and entirely new function for the first time. The work, which converted fatty acid decarboxylase (FAP) into new-to-nature radical photocyclases that catalyse the formation of carbon rings, demonstrates how photoenzymes can be evolved and repurposed for biocatalytic applications.
FAP enzymes, which are activated by blue light, were first discovered in 2017 in green microalga and shown to produce hydrocarbons by catalysing the removal of carboxyl groups from long-chain fatty acid substrates. As one of only three known classes of natural photoenzymes, researchers have been keen to exploit FAP’s potential and produce environmentally-friendly natural products, including carbon-neutral hydrocarbons for fuels, chemicals and cosmetics.