Longer-lived oxides offer silicon synthesis boost
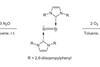
Touted as ‘soluble sand’, stabilised small silicon oxides present new synthetic worlds

Touted as ‘soluble sand’, stabilised small silicon oxides present new synthetic worlds