Longest silicon—silicon double bond has two-faced reactivity
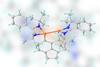
First hypercoordinated disilene’s extra-long silicon–silicon double bond gives it ambivalent reactivity
Chemists have broken the record for the longest silicon–silicon double bond ever made. This bond gives the molecule featuring this elongated connection ambivalent reactivity: it can either react as a double bond or as if it had two divalent silicon atoms.
