Scientists in the US have shown how a mechanochemical method – liquid-assisted ball mill grinding – can be used to functionalise post-consumer polystyrene. The team behind the work suggest it could be developed to upcycle commercial polymers by altering the polymer backbone. But other researchers are sceptical that such a process would ever be truly useful or sustainable.
Packaging materials, signs, and disposable cutlery and plates are some of the many single-use items commonly made from polystyrene. But because it needs to be sent to a specialised facility, recycling rates for polystyrene are low. Moreover, it’s often contaminated with food, which adds further complications.
Upcycling polymers, particularly through post-polymerisation methods, is one alternative to recycling them. Post-polymerisation methods involve functionalising the polymer backbone to endow the polymer with new characteristics and improve properties such as surface wettability, adhesion and thermal performance. However, such methods are limited because they require the complete dissolution of higher molar mass polymers and large volumes of solvent.
One emerging method that circumvents these issues is solid-state ball mill grinding. However, ball mill grinding is limited to lower molar mass polymers as it damages high molar mass polymer backbones, resulting in a loss of bulk properties due to polymer chain scission. It therefore has rarely been applied to post-consumer polymers.
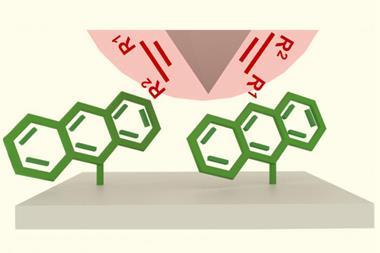
Now, a team around Matthew Golder at the University of Washington has used liquid-assisted grinding to functionalise a polystyrene backbone with fluorene, thereby increasing the polymer’s hydrophobicity making it potentially useful in coatings and dielectrics. Liquid-assisted grinding is an extension of traditional mechanochemical ball-milling techniques and uses a small amount of liquid to control reactivity.
The Washington team focused on fluorine functionalisation as they could easily quantify the reaction using 19F NMR spectroscopy. ‘The trifluoromethyl group was unique enough compared to known chain end internal standards to allow us to really determine how much of this reaction was occurring relative to how much chain scission was occurring,’ explains Golder. This work, he says, was a ‘proof of concept’ and is ‘definitely many, many steps removed from some of the more applied end goals.’
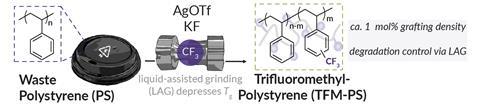
The team optimised various parameters of the liquid-assisted grinding ball-milling process to minimise degradation of the polystyrene polymer. They found that a plasticiser in consumer plastics reduced polymer chain scission. ‘[It] was an interesting finding, for specifically the high molar mass post-consumer waste plastic – the idea is that you wouldn’t really have to add much to post-consumer waste [to upcycle it],’ says Golder.
Far from a silver bullet
However, the team do note some shortcomings with the process, namely, the solvents. ‘The chemistry didn’t work in certain solvents,’ says Golder. ‘Dichloroethane worked really well. Unfortunately, dichloroethane is not the best solution in the world, especially if you’re thinking about scaling this,’ he says. ‘We’re thinking about ways that we can start to move away from halogenated solvents and potentially start to use bio-based solvent screen solvents etc within the context of these liquid-assisted grinding processes.’
‘Liquid-assisted grinding post-polymerisation is exciting because ball mills could potentially be driven by renewable energy,’ comments Ina Vollmer, an expert in chemical upcycling from the University of Utrecht in the Netherlands. ‘This new tool in the mechanochemistry toolbox of exploiting plasticisation effects is very welcome.’ However, she also notes her own concerns regarding the solvents. ‘Even though less solvent is used, it will need to be removed at the end. This could be a limitation as solvent recovery is an energy-intensive process.’
Others share Vollmer’s concerns. ‘The trifluoromethylation reactions on polystyrene have enormous environmental impacts due to the reagents used and if applied at scale could cause far more emissions than would be saved if polystyrene was prevented from being incinerated,’ says Anthony Ryan, a sustainable plastics expert at the University of Sheffield in the UK.
Moreover, Roman Boulatov, a mechanochemist from the University of Liverpool in the UK notes that the study doesn’t consider the cost of reagents, of their disposal or the energy needed to operate, or cool, the ball mill.
‘Solid state chemistry is intrinsically interesting and could lead to a number of specialised materials for niche applications. So [this work] is in itself a good thing,’ says Ryan. ‘[But] what do they think the potential demand for such upcycled products might be? How much polystyrene waste might be prevented?’
Vollmer reaches a similar conclusion: ‘An important aspect is the demand for the final product. What is the size of the market for such a specialty polymer? I estimate that the market size is rather small and therefore, post-polymerisation methods will likely address only small volumes of plastic waste.’
However, Golder remains hopeful and hopes to expand his team’s work. ‘We’re definitely interested in exploring more applicable transformations. Not only with polystyrene but with other post-consumer plastics such as polyolefins and figuring out how we can start to use these same principles.’
References
This article is open access
M E Skala, S M Zeitler and M R Golder, Chem. Sci., 2024, DOI: 10.1039/d4sc03362k












No comments yet