A Scandinavian collaboration has taken another step towards generating universal blood that is suitable for everyone receiving a transfusion or an organ transplant with the use of microbial enzymes. The enzymes come from a bacterium that feeds on mucus in the gut and can prune the A and B antigens from red blood cells.
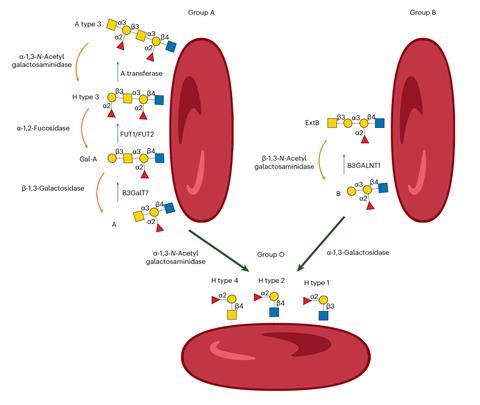
Blood types in humans are partly the result of the ABO gene coming in two forms, with the A gene producing one enzyme (N-acetylgalactosaminyl-transferase) and B another (α-galactosyltransferase). These enzymes add the A- and B-specific sugars to the glycolipids and glycoproteins on red blood cells. Those with O-type blood have neither enzyme and can donate blood and organs to all ABO blood groups.
Mismatched blood can cause lethal immune responses. ‘Everyone has natural antibodies against the antigens you miss,’ explains Martin Olsson, a blood expert at Lund University, Sweden, who co-led the new study. ‘If you tried to transplant a lung, liver or kidney against the ABO barrier, it would be destroyed within minutes.’
Almost 120 million units of blood are donated globally every year. Every unit must be blood-group matched with the patient. When a patient’s blood group in an emergency is unknown or cannot be determined, O-type blood is used. Demand for this universal blood type therefore outstrips supply.
In the early 1980s, a group at the New York Blood Center used α-galactosidase enzymes from coffee beans to modify B antigens to H antigens, which are those on O-type blood, a landmark advance. More recently, a screen of gut microbes identified an enzyme pair that can convert from A antigens to H antigens. None of these advances completely removed the cross-reactivity between blood groups, however.
Olsson says he now knows why. ‘We’ve uncovered new antigens linked to ABO,’ he says. ‘The textbooks were wrong. There are at least four other ABO-related antigens that cause the enigmatic reactivity when A and B are gone.’
These antigens can be pictured as extensions to the glycan tree on a red blood cell’s surface. Previous efforts to remove the glycan only trimmed the treetops. ‘We cut off the trees and for the first time see the shrubs and bushes underneath, which harboured these unknown antigens,’ says Olsson.
The enzymes were discovered in the lab of Maher Abou Hachem, a chemical engineer at the Technical University of Denmark, with a long-time interest in Akkermansia muciniphila. This gut microbe exclusively feeds on the densely glycosylated mucin, the main component of the mucosal layer lining our gut.
It turns out the glycans in mucin share similarities to those on red blood cells. ‘They are capped by terminal epitopes which resemble the blood antigen groups,’ says Abou Hachem, who co-led the research. ‘Both are gel-like and insoluble, negatively charged and complex glycan surfaces.’ After evaluating 22 hydrolases from the microbe, exoglycosidase combinations were identified that could efficiently trim A and B antigens and the four newly discovered carbohydrate extensions.
An estimated 95% of group O plasmas were compatible with group B blood converted with the new method, indicating that it may be acceptable for transfusion across the ABO barrier, says Olsson. Group A red cells are more complex and more work is needed to make enzyme-converted A blood that fits all patients.
This group’s work ‘led to significant compatibility improvements of treated red blood cells’, says Marcelo Guerin, a glycobiologist at the Institute of Molecular Biology of Barcelona. ‘These are truly outstanding discoveries that brings the [universal blood] concept much closer to clinical application.’
Biochemist Stephen Withers at the University of British Columbia, Canada, who has researched enzyme conversion of blood, says it will still take time to generate an approved universal blood product. ‘We all want to say it’ll be there in five years, but in reality I don’t think it will because safety studies take a long time,’ he says.
His group formed a company – Avivo – that is working with the US Food and Drug Administration on eliminating the blood-matching barrier. ‘They’re trying to understand what they would need us to do for a clinical trial,’ says Withers. ‘The regulatory agencies aren’t quite sure how to deal with this. This isn’t a normal drug, a biologic or a small molecule. Is it a medical device? They’ll need to decide.’
References
M Jensen et al, Nat. Microbiol., 2024, DOI: 10.1038/s41564-024-01663-4














No comments yet