Ancient alloy goes nano
Brass has been known to man since prehistoric times; now scientists in Germany have isolated the first molecular example of the copper–zinc alloy.1
The chemistry of solid state alloys is well established, yet understanding why different alloys possess particular properties is a greater challenge. Using a bottom-up approach, scientists aim to build intermetallic materials from the smallest available components, and identify boundaries where molecular properties meet bulk material properties. Constructing molecular clusters which mimic such materials is a step in this direction, providing fundamental insights into the chemical bonding of the target materials.
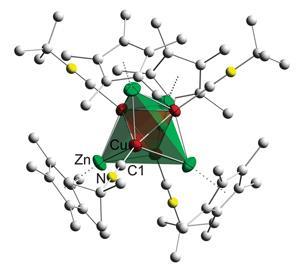
The challenge in synthesising such materials lies in finding the right precursor components, explains Roland Fischer: ‘from a thermodynamic point of view this should all be possible, but how do we make it? The art is in finding the right entry door down the right pathway.’ Complexes bearing metal–metal bonds between first-row transition metals remain exceptionally rare, owing to weak bonding interactions as a result of poor d orbital overlap between the small metal centres. Exploiting a low valent organometallic zinc compound,2 in combination with a cleverly selected isonitrile-functionalised copper complex, Fischer and co-workers from Ruhr University Bochum, Germany now report the unprecedented isolation of a ligand protected copper–zinc cluster. What’s more, this Cu4Zn4 complex features the first molecular example of an unsupported copper–zinc cluster covalent bond.
The cluster comprises a tetrahedral arrangement of four copper atoms, where each face of the tetrahedron is capped with a zinc atom, giving rise to an outer zinc tetrahedron. In fact, the very inverse of this Cu4Zn4 tetrahedral star is found at the core of a well-known phase of the alloy, γ-brass, where an outer Cu4 tetrahedron surrounds an inner Zn4 tetrahedron. ‘Of most interest is that the core resembles a well-defined cut out of the structure of γ-brass, or more simply put, a piece of brass coated with ligands,’ says Cameron Jones, an organometallic expert from Monash University in Melbourne, Australia.
‘We are so happy to be able to show that there is a structural relationship between our molecule and the structural principles of the intermetallics’, says Fischer. ‘Now our Mount Everest is to find the right components to mimic systems which are known to possess advantageous catalytic properties, and explore a whole area of untouched ground with respect to the reactivities of such compounds.’
References
1 K Freitag et al, Chem. Commun., 2014, DOI: 10.1039/c4cc03401e (This paper is free to access until 13 August 2014.)
2 I Resa et al, Science, 2004, 305, 1136 (DOI: 10.1126/science.1101356)











No comments yet