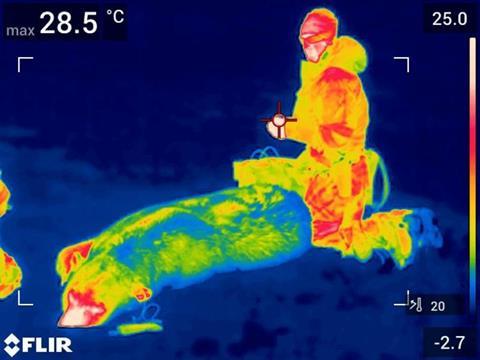
Polar bears stop ice forming on their fur thanks to the unique composition of their sebum that coats each hair. The discovery could inspire biomimetic alternatives to PFAS-containing ice-resistant coatings currently used.

Polar bears are the only land mammal that willingly dives into frigid Arctic waters. While their thermally insulating blubber and fur layers keep them warm, the surface of their fur is typically close in temperature to the ambient air. Despite this, polar bear fur does not accumulate ice – a property Inuit peoples have long used to make ice-resistant clothing, as well as hunting stools that can slide noiselessly across ice. The hairs of other semi- and fully aquatic animals, including humans, are coated in sebum, a complex mixture of lipids. The chemical composition of polar bear sebum, however, has not been studied until now.
Comparing fur samples collected from animals in Svalbard, Norway, an international team of researchers first identified that polar bear fur showed similar hydrophobicity to human hair and fluorocarbon-treated ski skins. However, when testing the ice adhesion strength of the materials differences emerged.
Surfaces with ice adhesion strengths below 100kPa are considered icephobic. Unwashed polar bear fur showed an average ice adhesion strength of 50kPa, roughly 40kPa lower than ski skins. But, when washed with a detergent to remove lipids, the ice adhesion strength rose by 100kPa. This trend was not mirrored in human hair, which showed an adhesion strength greater than 150 kPa both when washed and unwashed.
The scientists next investigated the molecular composition of polar bear sebum. Their analysis revealed a variety of lipid species, with waxes and glycerol species constituting the bulk, but, as opposed to all previous studies of mammals living in wet environments, no squalene.
The ice adsorption energies of the most abundant molecules – triglycerides, diglycerides, cholest-5-en-3β-ol and eicosanoic acid – were then evaluated using density functional theory, alongside PFAS and squalene for comparison. Researchers found that cholest-5-en-3β-ol and eicosanoic acid showed particularly low ice adsorption energies, comparable to those of PFAS. Squalene, on the other hand, had a high ice adsorption strength similar to unwashed human hair.
Attempts to elucidate the effects of lipid branching and degrees of saturation on ice adsorption energies found only a minor reduction in adsorption energies compared with unbranched lipids, with methyl-branched anteiso fatty acids being notable for their low adsorption energies. While increasing saturation in triglycerides followed a trend of lowering ice adsorption energies, diglycerides did not.
The researchers write that further investigation of the effects of methyl branching and saturation on lipid anti-icing properties, polar bear sebum could inspire environmentally friendly anti-icing materials.
References
J Carolan et al, Sci. Adv., 2025, DOI: 10.1126/sciadv.ads7321














No comments yet