Nanotube locked inside a porphyrin
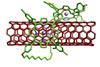
Rotaxane-like assembly formed from mechanically interlocked carbon nanotubes and macrocylic porphyrin rings
Scientists have created the first rotaxane-like assembly between carbon nanotubes and porphyrin rings, unlocking a new class of material for applications in catalysis, magnetism and sensing.