New class of recyclable permanently porous liquid doesn’t need a solvent
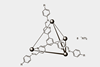
Coordination cages that are liquids at room temperature may find uses in catalysis, sensing or chemical separations
A new type of permanently porous liquid based on metal–organic cages has been created. The materials are recyclable and can encapsulate both gaseous and non-gaseous guests, which makes them attractive for catalysis and chemical separation.
While permanent porosity is a property that is usually associated with solids, in recent years scientists have been able to engineer persistent cavities into liquids using different approaches. Until now, the applications of these ‘permanently porous liquids’ have focused on gas storage and separation – and most studies have been limited to the binding of small molecules such as carbon dioxide and methane – so porous liquids with holes that can host more diverse guest molecules are in demand.