New form of pure carbon made by manipulating atoms
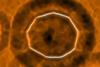
Elusive 18-carbon ring pinned down on surface
A new molecular form of pure carbon has been created that is neither a fullerene nor a relative of graphene. The IBM team produced a circle of 18 carbons using atom manipulation and analysed its structure with atomic force microscopy (AFM).
