New process draws poison from nitrile production
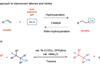
Nickel and aluminium catalysts obviate need for explosive and toxic hydrogen cyanide used to make important precursors for pharmaceuticals, cosmetics and agrochemicals

Nickel and aluminium catalysts obviate need for explosive and toxic hydrogen cyanide used to make important precursors for pharmaceuticals, cosmetics and agrochemicals