Non-metal catalyst reduces carbon dioxide to liquid fuels
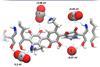
Conductive polydopamine channels electrons far more selectively than metal catalysts
The conversion of carbon dioxide to useful chemicals such as fuels could potentially close the anthropogenic carbon cycle. Now, researchers have unveiled an efficient, selective and metal-free catalyst for the reaction.