Odd 'entropic bonds' akin to chemical ones can form between nanoparticles
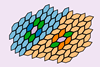
Simulations finds that ’bonding’ can occur in the absence of any electronic interaction
Entropy maximisation can lead to ‘entropic bonding’ that is remarkably similar to traditional chemical bonding between molecules, researchers in the US propose, even when effectively no electronic interaction occurs. The phenomenon could be useful in controlling self-assembly of colloids and other structures in soft matter.
