Crystalline microporous solids are an important class of inorganic material that impact our everyday lives. Their ordered structures contain arrays of channels and voids several nanometres across, enabling them to selectively and reversibly absorb molecules based on their shapes and sizes. This has led to their widespread use as catalysts, molecular sieves and gas sensors. Research into their potential use as hydrogen storage materials for mobile energy applications is also ongoing.
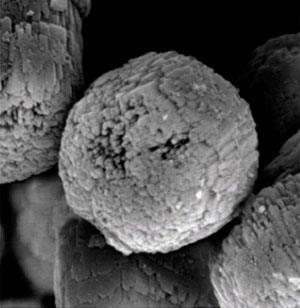
However, microporous solids often crystallise slowly and typically require several hours to several weeks of hydrothermal treatment to achieve satisfactory yields, limiting their applications on industrial scales. Now, a collaborative effort from the University of Tokyo and the Mitsubishi Chemical Group has led to an ultra-fast method for preparing the aluminophosphate AlPO4-5. A combination of rapid heating and crystal seeding completes the synthesis within one minute.

The team use a cheap stainless steel tubular reaction vessel that provides a very high surface area to volume ratio. ‘It takes only several tens of seconds to reach the target temperature in our tubular reactor compared to several hours for a conventional autoclave. This fast heating induces a higher crystal growth rate,’ says Tatsuya Okubo, from the University of Tokyo, who led the study. Adding a seed crystal to the reaction was also found to dramatically decrease the time required for crystallisation by bypassing the energy barrier for spontaneous nucleation of initial particles. Okubo’s team have applied this technique to develop a low cost continuous flow process with very high throughput, to meet industrial requirements for these materials.
‘Besides reducing the manufacturing cost of powders, fast growth can lead to rapid membrane synthesis and even provide a better mechanistic picture of crystallisation,’ says Prabir Dutta, a microporous materials expert at Ohio State University in Columbus, US. He adds that ‘the challenge is to see if this method can be generalised for the growth of other materials, especially the industrially important aluminosilicates’.






No comments yet