Perfectly planar stable silicon clusters with six contacts predicted
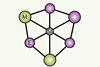
Hexacoordinated systems theorised to remain stable, even with protecting ligands
The first planar hexacoordinate silicon clusters found computationally as true global minimum forms have been reported by researchers in China, Germany and Mexico.
