Pick and mix macromolecules
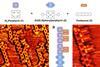
Activating C-H bonds in molecular building blocks enables complex macromolecules to be created

Activating C-H bonds in molecular building blocks enables complex macromolecules to be created