Ultrasmall chemical reactors have been created at junctions between crossed polymer nanofibres
Scientists in the US have taken inspiration from a Dutch painter to create ultrasmall chemical reactors at the junctions of overlapping polymer nanofibres. Their method could eventually be used for high throughput testing of new organic reactions - for as little as seven pence per reaction.
Pavel Anzenbacher Jr and Manuel Palacios, at the Bowling Green State University, have demonstrated that they can incorporate reagents between the threads of separate polymer nanofibres using a well-known technique called electrospinning. These nanofibres can then be laid on top of each other in a mat-like fashion, and the reagents at the ’junctions’ activated using heat or solvent vapour. Once activated, the different reagents in the two fibres react with each other to form a product that remains infused within the polymer fibres.
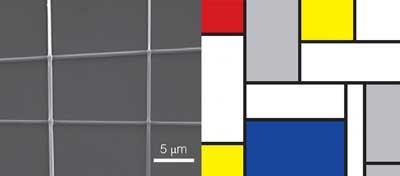
The team have coined these junctions zeptomole-scale chemical reactors - a zeptomole is a billionth of a trillionth (10-18) of a mole and Anzenbacher says that they are reacting just a few hundred molecules at a time. But he adds that ’in the future we think that we will be able to do a single reaction involving just one molecule from each nanofibre.’
Working on a small scale is very important for the chemical industry - for both cost and safety reasons - says Anzenbacher. You don’t want to produce more product than is needed when testing new reactions and new molecules, he explains, as the more solvent and reagents you use the more the reaction costs. Additionally he says, ’the polymers and the electrospinning method used to make the polymer fibres are very cheap.’ ’Because you are making such a small amount, and you know where the product is located, it is very easy to handle a potentially toxic product safely,’ he adds. ’There are micro- and nano-fluidic devices currently available that can be used to carry out reactions on small scales, but in most cases not this small.’
The team found that the reaction products can be analysed whilst still encased in the polymer nanofibres. We analysed the fibres directly with a mass spectrometer, explains Anzenbacher. They also demonstrated that the products can be easily extracted from the polymer. He explains this concept as being like making a cup of tea using a tea bag, so when the polymer is dipped in solvent the product (the tea) infuses out and the polymer (the bag) remains intact.
Getting inspired
Anzenbacher says that the work of the Dutch artist Piet Mondrian was their inspiration for this work - and you can certainly see the similarities (see images). So far they have tested their concept on a range of different polymers - such as protein and organic polymers - and five different types of reactions, including nucleophilic and electrophilic substitutions, and have concluded that it be applied to a wide range of reactions.
The team are keen to promote their work as ’nanochemistry for the masses’ because it is easy to do and no expensive equipment is needed. ’We are planning to use it to get kids excited about nanotechnology and nanoscience,’ says Anzenbacher.
As well as inspiring the younger generation, the team are planning to take their work in two different directions. ’We have realised that we can do this on proteins and now we want to start working on biomolecules. We also want to test high throughput methodologies for testing organic reactions on this small scale,’ Anzenbacher says. Each reaction should cost just 10 to 20 cents (7 to 14 pence), he adds.
Robert Wootton an expert in microreactors at Liverpool John Moores University, UK, says that ’this research is very exciting’. We’ve had single molecule detection for a while but have not got down to such a small number of molecules synthetically, he says. ’This method certainly has a possibility of making it into the high throughput arena. Because the high throughput world is dealing with solid supported chemistry already and this is a reaction that is taking place between what you could envisage as two solid supports,’ he adds.
Nina Notman
References
P Anzenbacher Jr and M Palacios, Nature Chemistry, 2009, DOI:10.1038/NCHEM.125






No comments yet