New computational studies could help scientists unpick the knots in real proteins
A new study may help scientists unravel the complex problem of protein folding. The study suggests knotted proteins, which present a particular challenge to folding experts, could be untied with a couple of well-targeted tugs.
Protein function is intimately related to protein folding. The folding process, however, remains something of a mystery and due to its speed is almost impossible to observe. Knotted proteins, discovered relatively recently, are puzzling because it is unclear how they could fold through any of the routes so far identified. Experimentally, it’s also difficult to tell whether a protein that has been denatured is still knotted - or not.
Joanna Sulkowska at the University of California-San Diego in the US and colleagues have now employed computational methods to show that knots in methylotransferase proteins could be unpicked by pulling on exactly the right residues. Using simple equations, they pinpoint the knot and its key residues relative to the protein’s ends. Their molecular dynamics simulations support the idea that pulling in the right places will undo the knot.
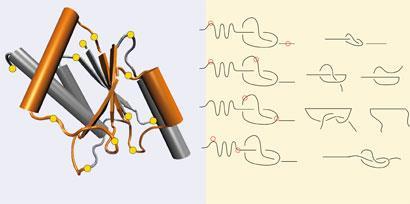
’Up to now there was no way to unfold the protein and this paper suggests that, yes, you can untie it, but you need to pull in very particular spots,’ says Sulkowska. ’Then you can study the full folding mechanism.’ The right spots, Sulkowska explains, could act as attachment sites for cantilever tips, which could then be used to untie a knot under an atomic force microscope. She says the method should apply to all knotted proteins so far discovered.
’The work will be important for the interpretation of mechanical unfolding studies,’ says Sophie Jackson, a protein folding expert at the University of Cambridge, UK. But she adds that mechanical unfolding is a ’special case’ - unfolding in thermal and chemical denaturation experiments proceeds by a different route.
Sheena Radford, who studies folding mechanisms at the University of Leeds, UK, thinks the work is intriguing, but leaves plenty to the imagination. ’Although it allows us to see whether you could unknot a protein when you pull it in different directions, I don’t think it answers the question that for me is most fascinating, which is how biology ever knotted it in the first place.’ It’s like asking a three-year-old to untie a shoelace, she says - that may be easy, but re-knotting it will be much harder.
Hayley Birch
References
et alJ. Am. Chem. Soc.DOI: /ja102441z






No comments yet