Protein synthesis revolution on way as large peptides made in hours not days
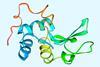
Flow chemistry can now make peptide chains up to 164 amino acids long in one go
A flow chemistry setup can synthesise small proteins that are more than three times larger than the polypeptide chains that can be made using conventional techniques.1 The approach is very fast and can be used to add both natural and unnatural amino acids at any position on the protein backbone, opening new possibilities in drug discovery and materials science.