Purple crystals boast first aluminium double bond
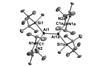
Aluminium analogue of an alkene completes series of double-bonded boron group elements
The first stable dialumene – a molecule with an aluminium–aluminium double bond – has been made in the form of intensely purple crystals. While double- and triple-bonded compounds of the other group 13 elements – boron, gallium, indium and thallium – are already known, the corresponding aluminium double bond has so far eluded scientists. Aluminium’s preferred oxidation state is +3; in a neutral dialumene, however, the metal would be in a +1 oxidation state.
