Quantum computing has provided new insights into a fundamental aspect of photochemical reactions that has previously proven difficult to study. The findings could improve scientists’ understanding of light-driven processes such as photosynthesis, smog formation and ozone destruction.
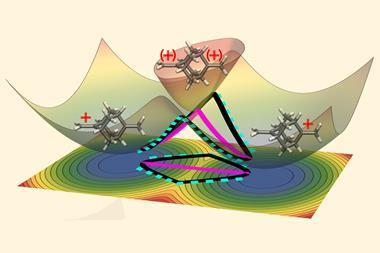
Photochemical processes occur when atomic nuclei and their electrons take on different configurations after absorbing a photon. Some of these reactions are guided by a quantum phenomenon called a conical intersection, where the potential energy surfaces that describe a molecule in its ground state and in its excited state converge. In these situations, quantum mechanical interference can prevent certain molecular transformations from taking place – a constraint known as a geometric phase. This limits the path that the reaction can take and affects the reaction outcome. The geometric phase has been known about since the 1950s, but due to the femtosecond timescales involved, it has never been directly observed in a molecular system.
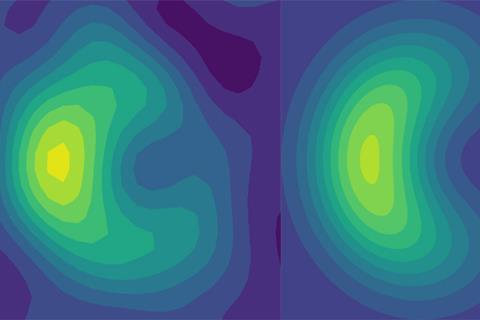
Now, two research teams working independently of each other have shown how the geometric phase can be measured using quantum simulators.
‘This phase is very hard to see in real molecules because it is happening far from the ground state and requires a clean quantum state with little thermal interference,’ says quantum systems researcher Kenneth Brown from Duke University in North Carolina, US.
‘We construct a quantum system that has some of the properties of the system we want to study,’ says Brown. The design of this quantum simulator allowed the Duke team to measure the effect ‘at a timescale which is much easier to read out’, says Brown.
The researchers used lasers to manipulate a chain of five trapped ytterbium ions in a manner that imitates the quantum behaviour of atoms at a conical intersection. As the quantum dynamics of the trapped ions are much slower than those of a molecule, the team was able to directly measure how the geometric phase affected the spatial distribution of an ion’s wavefunction.
‘Our experiment is one of the earliest demonstrations of how we can do electron vibrational coupling of trapped ions,’ says Brown. He notes that understanding the geometric phase could offer chemists ‘another way to control’ the products that are made during multi-product reactions.
The Duke team’s findings were published alongside similar work led by researchers at the University of Sydney, Australia. Thegroup, led by Ivan Kassal, used an analogue quantum simulator based on a single trapped ytterbium ion.
‘One of the most important things here is that we were able to observe, in real time, the geometric phase interference in this system that behaves as fast as [a] molecular system,’ says Vanessa Agudelo, a PhD student in Kassal’s lab who worked on the project.
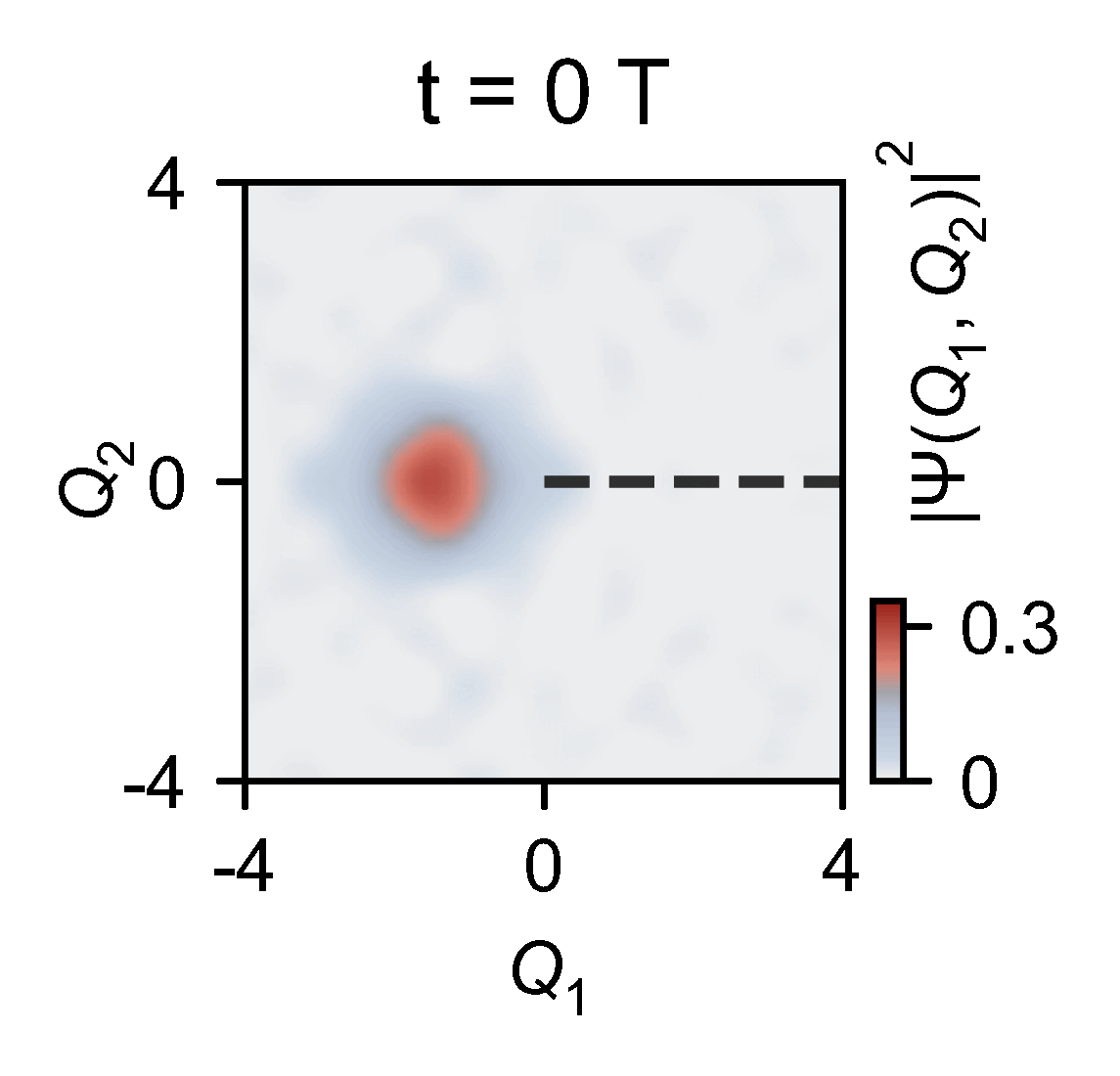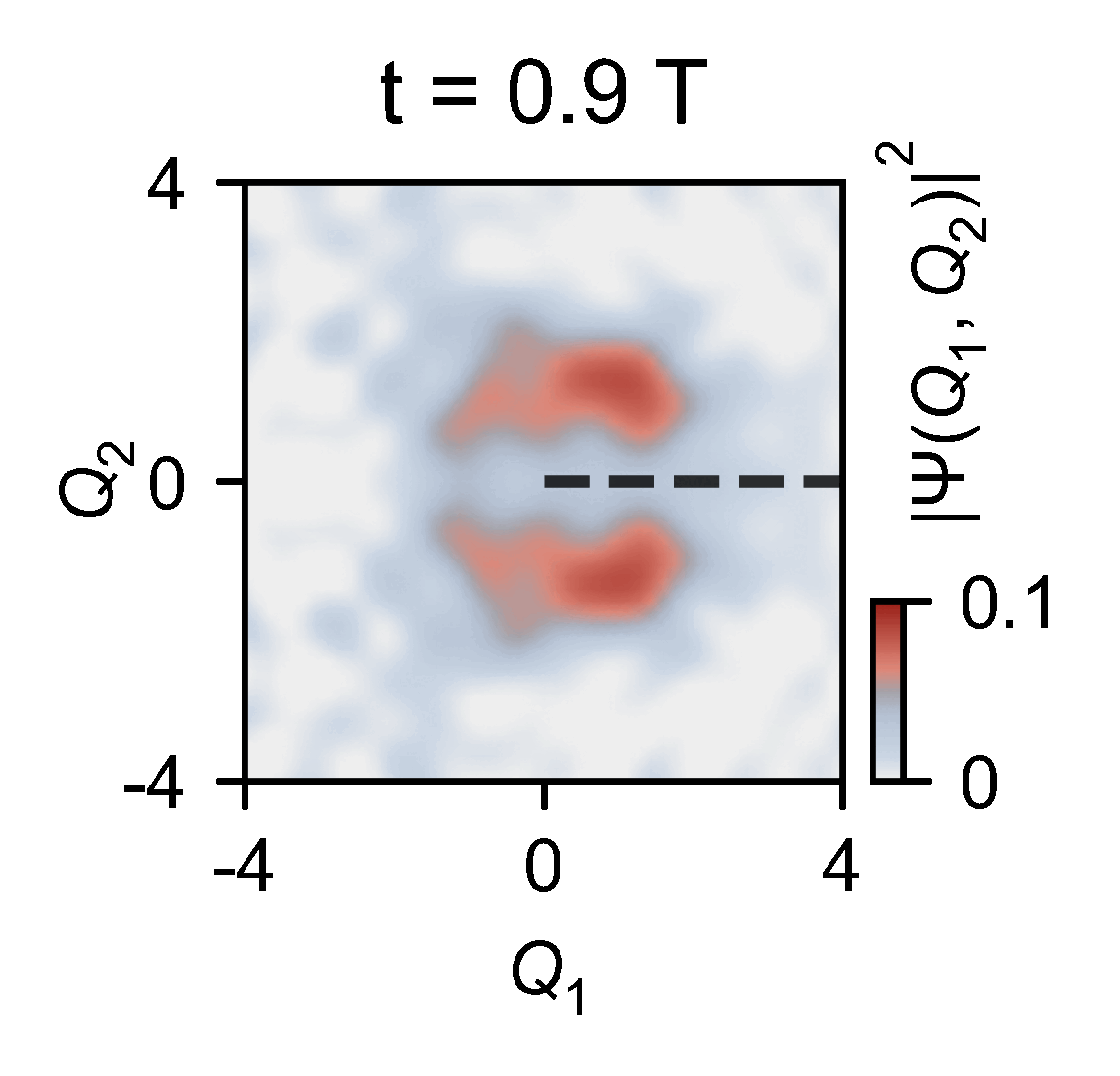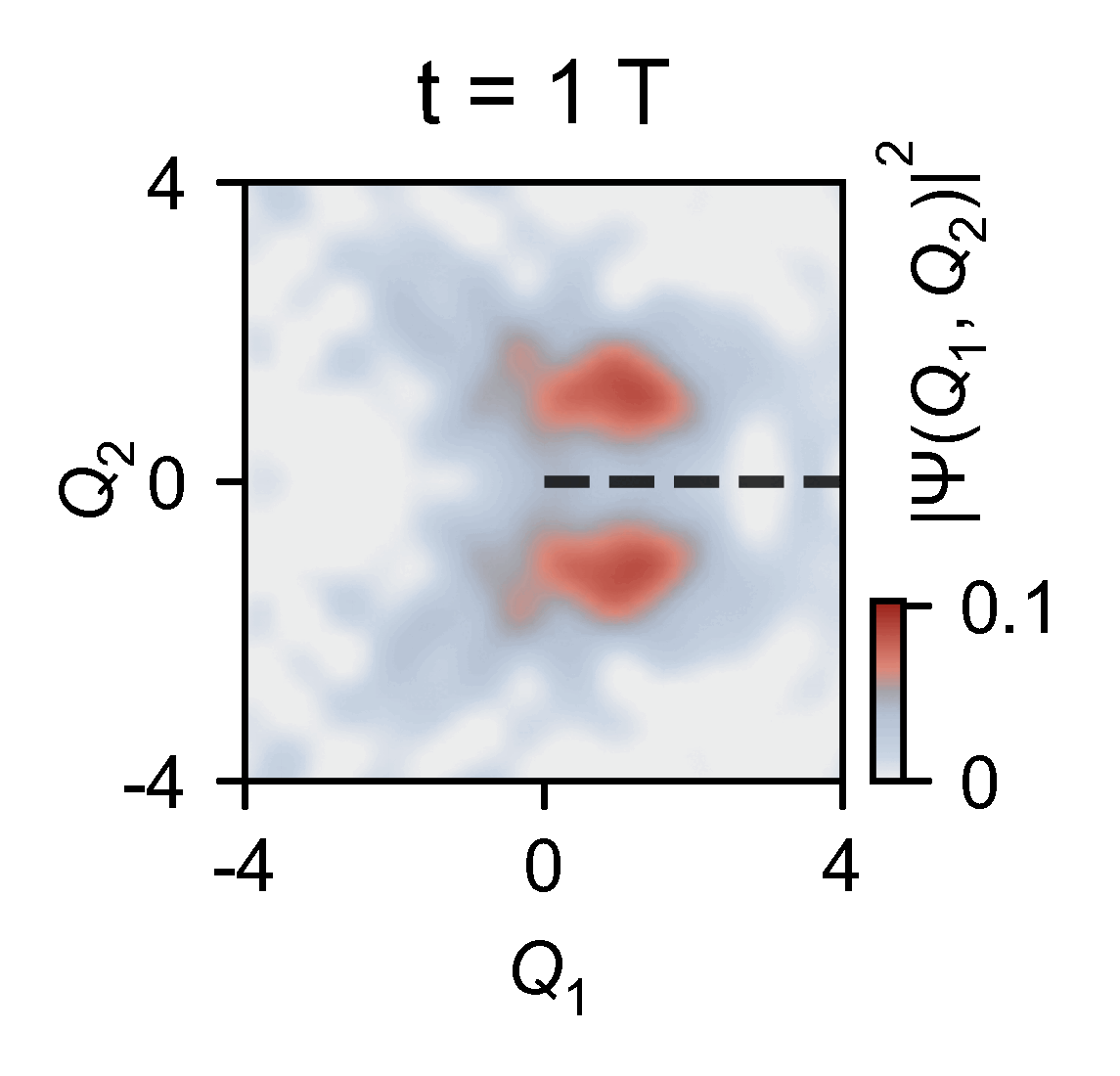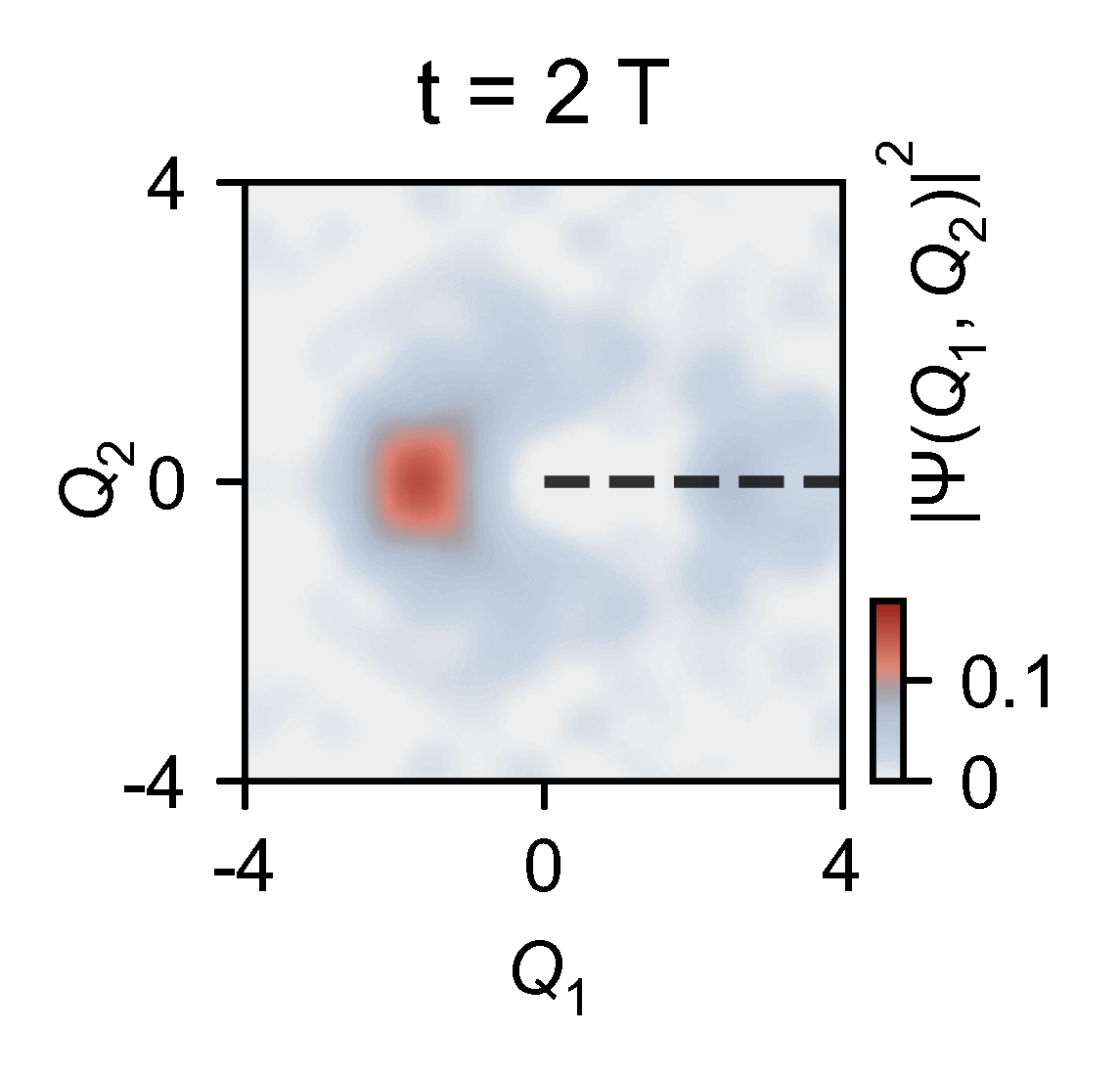
The quantum computer allowed the them to slow down the chemical dynamics of the system they were studying from femtoseconds to milliseconds, enabling meaningful observations.
‘Here you have an actual video of a single atom splitting in half, … interfering with itself destructively when it goes on the other side of the conical intersection,’ explains Kassal. ‘This is simulating a photon coming in and how the molecule interacts on femtosecond timescales.’
‘That’s particularly important in things like atmospheric chemistry – why does smog form? How does the ozone layer form? Or how is it destroyed?’ he adds.
While the technical approach taken by the two teams differs, their findings are consistent. Kassal notes that the work underscores how quantum computing can help to solve complex chemistry problems. ‘The goal of using quantum computers for chemistry is to be able to simulate any kind of chemical process … like drug discovery, or the discovery of better materials,’ he says.
References
J Whitlow et al, Nat. Chem., 2023, DOI: 10.1038/s41557-023-01303-0
C H Valahu et al, Nat. Chem., 2023, DOI: 10.1038/s41557-023-01300-3












No comments yet