Radical ion synthesis finally embraces green chemistry
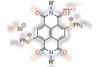
Mechanochemical route to naphthalenediimide radical ions
Researchers in India have devised the first eco-friendly method for generating organic radical ions.

Mechanochemical route to naphthalenediimide radical ions
Researchers in India have devised the first eco-friendly method for generating organic radical ions.