Record-breaking molecular nanoribbon exhibits unique optoelectronic properties
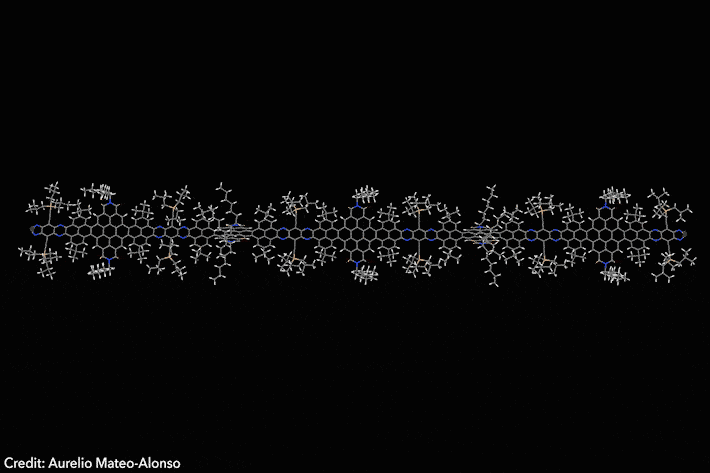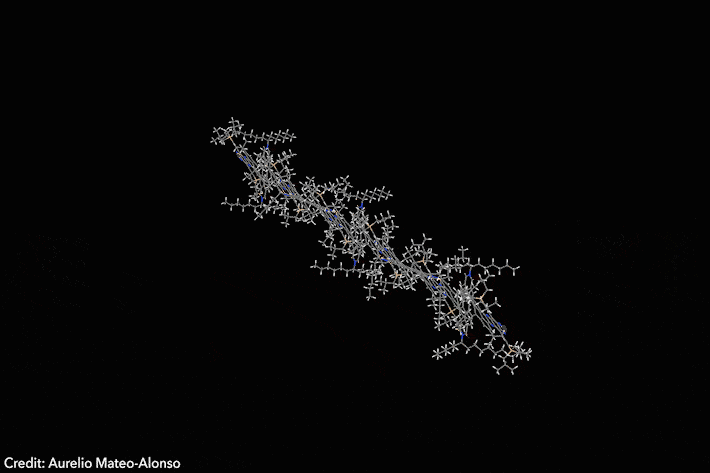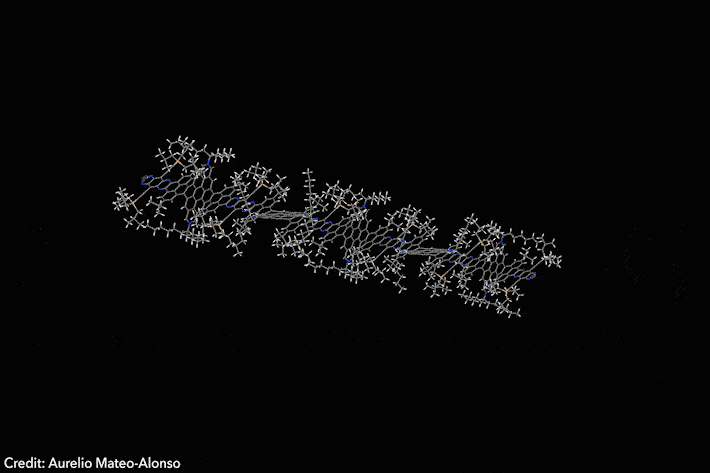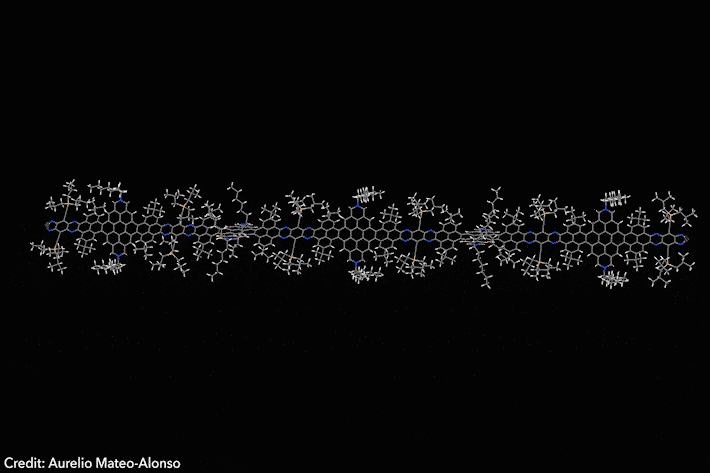
Assembled sequence of 53 aromatic rings has surprisingly high solubility
Four years ago, chemists developed a Lego-like methodology to prepare giant molecular aromatics. Now, the same team has broken all the records by crafting a huge twisted nanoribbon almost 13nm long, featuring 53 linearly fused rings and over 320 conjugated atoms. The molecule also exhibits exciting optoelectronic properties.1
‘Making giant aromatics is a very difficult task,’ explains Aurelio Mateo-Alonso of Polymat in Spain, who led the study. ‘Large aromatic structures pile-up into stacks, which renders them insoluble and nearly intractable materials that cannot be purified and characterised with traditional techniques,’ he adds. Twisted aromatics include sterically crowded moieties that force them into curled arrangements, making them less prone to form stacks and generally more soluble than their planar counterparts.
