Redox switches up possibilities for signal transduction in artificial cells
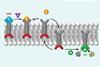
Vitamin C triggers membrane translocation in a synthetic system, which generates an amplified fluorescent response
Chemists in the UK have developed a new artificial system that mimics biological transmembrane signalling. Using a membrane-anchored molecule in the phospholipid bilayer of a synthetic cell as a sensor, a redox signal outside the cell can generate an amplified fluorescent response inside it.