Salting out artificial photosynthesis
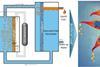
Photoelectrochemical system theorised to produce high concentrations of liquid ethanol from carbon dioxide

Photoelectrochemical system theorised to produce high concentrations of liquid ethanol from carbon dioxide