Scientists poke holes in zeolite theory
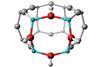
Computational studies challenge aluminium avoidance rule
Theorists in the UK have studied the aluminium distribution in a number of catalytically active zeolite species, finding evidence that –Al–O–Al– linkages could exist in some zeolite species after all.