Seed oil-based polymer should survive a day in the rain but degrade within years in the sea
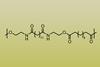
Researchers create polyesteramides from brassylic acid and explore their potential as a replacement for polyethylene
Researchers have used chemicals derived from inedible seed oil to synthesise water-degradable polymers. And while the polymers degraded significantly when left in seawater for a year, they stayed strong and flexible after being wet for just one day.
