Self-tying trefoil knot
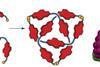
UK scientists have stumbled across a molecule that ties itself up in knots, echoing the folding of proteins

UK scientists have stumbled across a molecule that ties itself up in knots, echoing the folding of proteins