Shining a light on amine synthesis
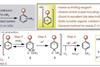
Photoredox catalysis enables site selective amination and could make production of anilines safer

Photoredox catalysis enables site selective amination and could make production of anilines safer