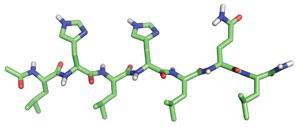
The peptides self-assemble into fibrils that resemble amyloid protein aggregates that are present in neurological diseases such as Alzheimer’s. The new finding hints that these amyloid structures could have more a complex biochemistry than previously realised.
A team led by William DeGrado at the University of California, San Francisco and Ivan Korendovych at Syracuse University constructed seven-residue peptides with alternating hydrophobic amino acid residues that self-assemble into sheet-like structures and aggregate into fibrils. In the presence of zinc ions, the fibrils were able to catalyse the hydrolysis of a model ester, p-nitrophenyl acetate.
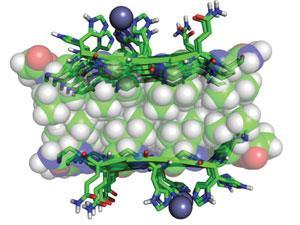
Because the fibril structures are reminiscent of the amyloid plaques that are present in certain human brain diseases, it is conceivable that naturally occurring fibrils have a more complex chemistry than previously thought, says Korendovych. ‘It might be worth investigating if catalytic activity might contribute amyloid toxicity,’ he suggests.
Louise Serpell, who researches amyloid fibrils at the University of Sussex in the UK, describes the study as ‘really impressive’.
‘Enzymes have evolved to be very large so that they can accommodate an active site, and there have been many attempts in the past to make minimal protein structures with catalytic active sites, but they pretty much don’t work,’ she says. ‘What this new study shows is that it is possible to make a catalytic protein structure out of much smaller amyloid type peptides, which is really amazing.’
Serpell adds that while the possibility that amyloid fibrils involved in human in human diseases such as Alzheimer’s may display catalytic activity is intriguing, the current study does not provide sufficient evidence that this is the case. ‘But it may be something that is worth investigating further,’ she says.






No comments yet