Simplified hydroformylation replaces rhodium with base metal
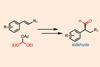
Bench-friendly asymmetric hydroformylation swaps toxic gas and expensive catalyst for cheap reagents and mild conditions
A bench-friendly alternative to traditional hydroformylation replaces the pressurised toxic gas mixture and expensive rhodium catalyst with a commercially available electrophile and cheap copper reagent. Through careful choice of ligand, US-based researchers closely controlled the stereoselectivity of the reaction to generate a range of valuable aldehydes for synthesis and drug discovery.
