Stable polynitrogen synthesis first blows everyone away
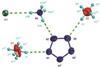
Creation of five-membered aromatic nitrogen ring opens door to new highly energetic rocket fuels and explosives

Creation of five-membered aromatic nitrogen ring opens door to new highly energetic rocket fuels and explosives