Solving the structure of viral nucleoprotein could boost quest for new drugs
The crystal structure of a molecule important for the replication of the influenza A virus has been solved, report US researchers. Now that scientists know what the protein looks like they can design drugs that block its action and prevent viral spread through the body.
Influenza A viruses, including the avian flu virus, H5N1, are a global health threat. Since 1900, different strains of these microbes have been responsible for three major pandemics that killed over 50 million people worldwide.
For a viral particle to survive and cause disease, it must make more copies of itself by hijacking a host cell and replicating its own genetic information. The genetic material of influenza A viruses exists in a complex of RNA and protein called the ribonucleoprotein. The backbone of this molecule - the nucleoprotein - plays a critical role in the replication of the viral RNA genome.
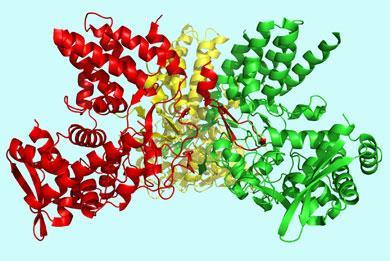
Jane Tao and colleagues at Rice University in Houston, Texas and the University of Texas, Austin now report the crystal structure of the influenza A nucleoprotein. Previous attempts to crystallise the protein failed because it tends to form different sized polymers.
’To grow crystals you need a homogeneous group of molecules,’ Tao, a structural virologist, told Chemistry World. ’We tried our luck with different nucleoproteins from different influenza A virus strains,’ she explained. ’Eventually we found one that could be crystallised.’
Analysis of the diffraction pattern of the nucleoprotein crystals revealed a molecule that resembles a ’crescent with two domains - a head domain and a body domain and a little tail at the back of the molecule,’ said Tao.
’It looks like a Pac-Man with a tail,’ said Paul Digard, a molecular virologist who studies influenza virus replication at the University of Cambridge, UK.
Tao’s team showed that the tail of the molecule interacts with a cavity in adjacent nucleoprotein molecules. When Tao mutated the tail, the nucleoprotein lost the ability to form polymers with other nucleoproteins. Since self-association of nucleoproteins is necessary for viral replication, compounds that interfere with the function of the tail could provide new drugs for treating flu.
’It’s good to come up with new antivirals because some strains, including some H5N1 viruses, are resistant to [the common flu drug] Tamiflu,’ said Tao. To that end, Tao and colleagues are screening compounds to identify drugs that inhibit viral replication by interfering with the tail-binding pocket.
’This paper describes a wonderful bit of work that helps put a lot of previous molecular biology on the influenza nucleoprotein into perspective,’ said Digard. ’Like any crystal structure, however, it’s a static snapshot of one conformation of nucleoprotein and I think there may be further surprises as we get more information on what the protein looks like bound to RNA.’
Jessica Ebert
References
Q Ye, R M Krug and Y J Tao, Nature, 2006 DOI:10.1038/nature05380






No comments yet