Study finds Goldilocks organoborane catalysts for fluorination
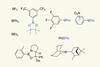
Tuning fluoride ion affinity opens a window for organoborane phase transfer catalysis in nucleophilic fluorination reactions
UK-based researchers have found a family of organoborane catalysts that can perform nucleophilic fluorination reactions. While chemists are well-accustomed to the high fluorophilicity of organoboranes, looking more closely into the different degrees by which they exhibit that characteristic affinity reveals that some of them could serve as fluoride phase-transfer catalysts.